
In short, Thomson had discovered the existence of particles smaller than atoms. How did Thomson disprove Dalton’s theory? The current model of the atom includes protons, neutrons, and electrons.

As these particles moved away from their original atoms, they formed a visible beam.
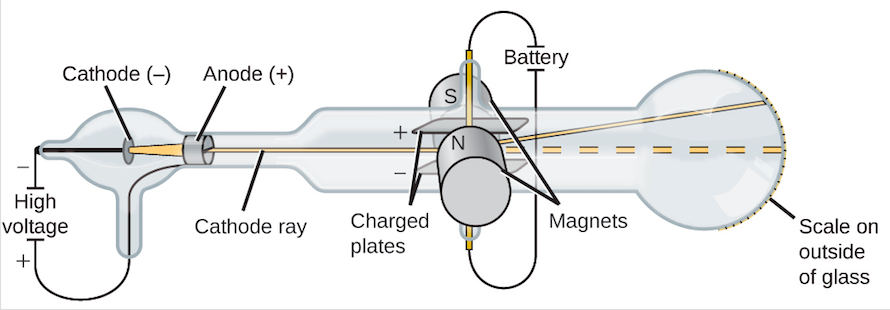
The charged particles in the beams that Thomson studied came from atoms. What did he figure out the beam was actually made of? : cathode-ray tube also : a display device incorporating a cathode-ray tube. Rutherford model, also called Rutherford atomic model, nuclear atom, or planetary model of the atom, description of the structure of atoms proposed (1911) by the New Zealand-born physicist Ernest Rutherford. Johnstone Stoney to denote the unit of charge found in experiments that passed electrical current through chemicals it was Irish physicist George Francis Fitzgerald who suggested in 1897 that the term be applied to Thomson’s corpuscles.) Which gas is used in cathode ray experiment?įor better results in a cathode tube experiment, an evacuated (low pressure) tube is filled with hydrogen gas that is the lightest gas (maybe the lightest element) on ionization, giving the maximum charge value to the mass ratio (e / m ratio = 1.76 x 10 ^ 11 coulombs per kg). (The term “electron” was coined in 1891 by G. Rutherford’s gold foil experiment showed that the atom is mostly empty space with a tiny, dense, positively-charged nucleus. Thomson’s experiments with cathode ray tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons. Thomson invented to test the theory that negative charges in an atom were real. Thomson is the scientist that discovered electrons through an experiment called the Cathode Ray Experiment. What did the cathode ray experiment discover quizlet? The term cathode ray is obsolete today the rays would be described as a beam of electrons. They were called cathode rays because they were emitted from the cathode of the vacuum tube. Why are they called cathode rays?Įugen Goldstein coined the term cathode rays in 1876. Thomson demonstrated the existence of the electron. Dalton thought that atoms were indivisible particles, and Thomson’s discovery of the electron proved the existence of subatomic particles. Thomson’s experiments with cathode ray tubes helped him to discover the electron (which Dalton did not know about). What was JJ Thomson’s cathode ray experiment? – Related Questions What did the JJ Thomson’s cathode ray tube experiment add to the atomic theory? This cylinder had two slits in it, leading to electrometers, which could measure small electric charges. His first experiment was to build a cathode ray tube with a metal cylinder on the end. What was JJ Thomson’s first experiment? Thomson’s First Cathode Ray Experiment Accordingly, he called his particles electrons. Thomson’s discovery established the particulate nature of electricity. What discovery did JJ Thomson’s cathode ray experiment lead to? With both magnetic and electric deflections observed, it was clear that cathode rays were negatively charged particles.
#Jj thomson cathode ray experiment explanation full
the rule that states that atoms tend to be the most stable when their valence shells are completely full normally, when the valence shell contains eight valence electrons. What did JJ Thomson’s cathode ray tube experiment show quizlet? Thomson’s cathode ray tube experiments provided the first evidence that atoms were composed of even smaller particles called electrons. Thomson proposed the plum pudding model of the atom, which had negatively-charged electrons embedded within a positively-charged “soup.”


What was JJ Thomson’s cathode ray experiment? Summary.


 0 kommentar(er)
0 kommentar(er)
